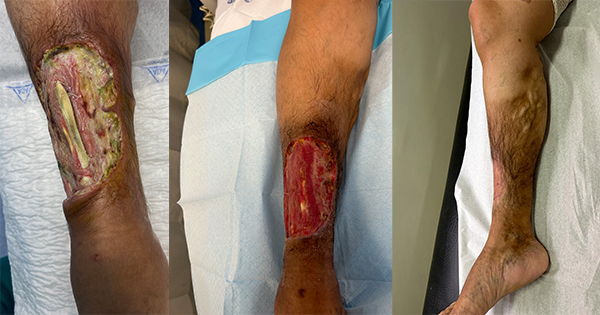
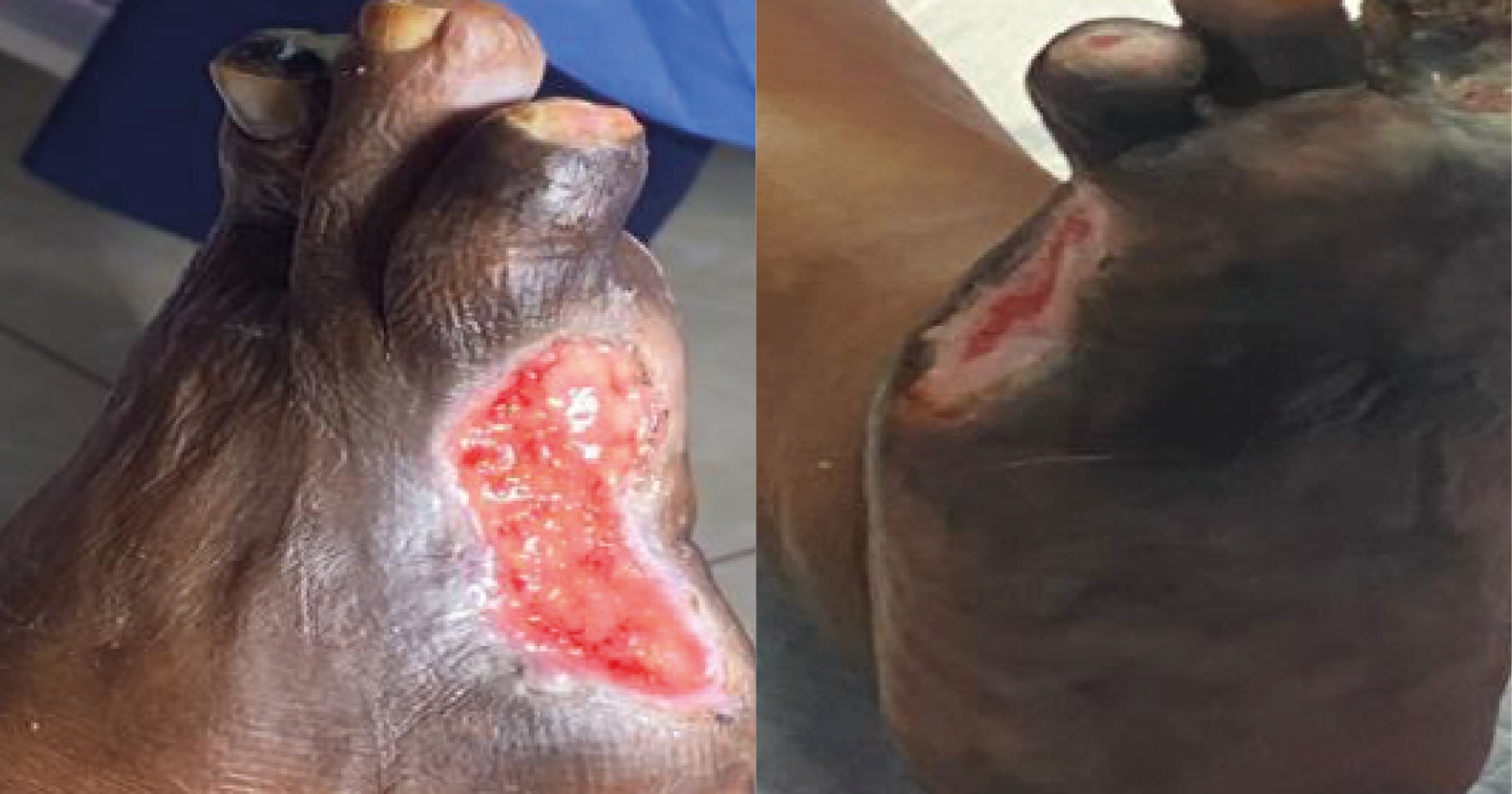
<p>Hard-to-heal wounds are challenging to treat and have a significant impact on a patient’s quality of life and healthcare resources [1,2,3]. For clinicians, hard-to-heal wounds pose the dual challenge of providing cost-effective management, while improving patients’ wellbeing and concordance with treatment [1,4,5]. This paper examines the impact of hard-to-heal wounds on patients and reviews the clinical efficiency and cost-effectiveness of a topical antibacterial dressing containing silver sulfadiazine (ALLEVYN™ Ag, Smith & Nephew) in the management of patients with infected hard-to-heal wounds.</p>